Glycerol is alcohols which contains three carboxyl. It has the chemical reactivity of general alcohols. Because he has three carboxyl groups, all glycerols have features of monohydric alcohols, diols and other different polyols. That the below chemical reaction of glycerin can explain the role of glycerin in chemical industry.
2.2.1 Oxidation of glycerin
Under normal conditions, glycerin is stable in the atmosphere, but it is easily oxidized by other oxidants. Glycerin can be oxidized under different conditions to produce different compounds.
- In the presence of ferrous salts, Glyceraldehydes are formed under mild conditions with hydrogen peroxide.

- glycerol can be oxidized by nitrite oxidation.

- With the action of Acetobacter SP, two carboxy acetone was formed.

- reaction with calcium carbonate and hydrogen peroxide to form carboxyl malonic acid.
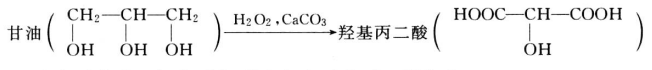
- Acrolein is produced by potassium hydrogen sulfate or potassium sulfate
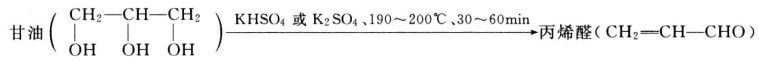
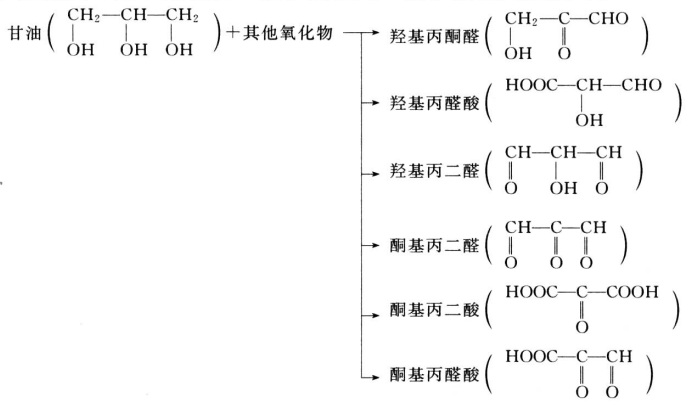
- Other oxides that can be produced by reacting with other oxides include carboxyl pyruvic aldehyde, carboxyl pyruvic acid, carboxyl malondialdehyde, keto malondialdehyde, keto malonic acid, keto pyruvic acid, etc.
- Periodic acid oxidation: Periodic acid is a selective oxidant that can react with 1,2-ethylene glycol to break the carbon chain between consecutive methanol and produce aldehydes or ketones, which can be carried out in neutral or acidic conditions. When reacting with glycerol, the carbon atoms break at both sides of the intermediate carbon atom to form formaldehyde, and one carbon atom in the middle to form formic acid. This response is quantified. Therefore, it is one of the main methods of glycerol analysis.
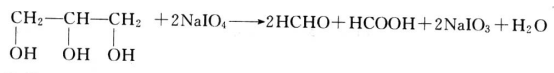
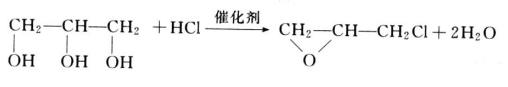
- Epoxidation of glycerol with hydrogen chloride by intramolecular dehydration in the presence of catalyst to produce epichlorohydrin. Epichlorohydrin is widely used and plays an important role in the generation of epoxy resin. This reaction is expected to become the most important component of glycerol derivatives.
2.2.2 Reduction of glycerin
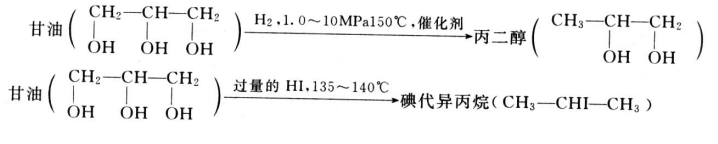
There is no industrial use of glycerin reduction reaction to produce other products, if the Ni, Fe, Pt, Au, Hg, etc. as a catalyst, at 150 degrees Celsius, with H2 reduction will produce propylene glycol. In addition, iodinated ISO propane is produced by reduction of excess hydrogen iodide at 135-140 C.
2.2.3 esterification
Organic esters of glycerol are the most common and widespread glycerol biotechnology. They can form esters with low carbonation and higher fatty acids. Because glycerol has three hydroxyl groups, it can react with fatty acids at different locations, so there are many varieties. There are mainly three kinds of esterification reaction of glycerol, that is, general alkyd esterification, alcoholysis and transesterification three kinds. In addition, there are reactions of glycerol halides with fatty acid salts and glycerol with acyl halides (or anhydrides) and so on (about fatty acid glycerides, the application of glycerol will be introduced in the chapter).
- Alkyd esterification is usually carried out under acid catalyst conditions. The reaction temperature is 200~230 C and the reaction time is about 3~8h.
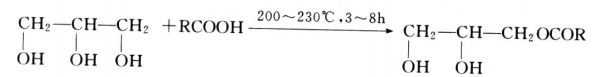
- The alcoholysis reaction is usually carried out under alkaline conditions. The reaction temperature is about 100 C and the reaction time is about 0.5~3h.
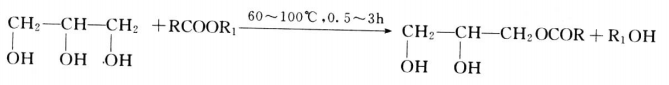
- Lipid exchange reaction
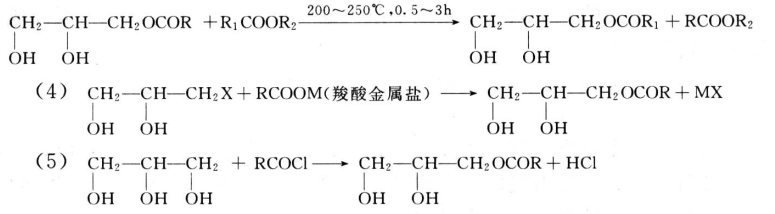
6.glycerol twelve acid (saturated or unsaturated) alkyd resin
2.2.4 Sulfation reaction
At 10~20℃, sulphuric acid can react with glycerol to react with 2~4h.
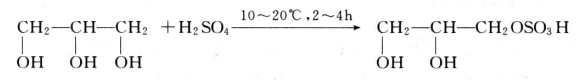
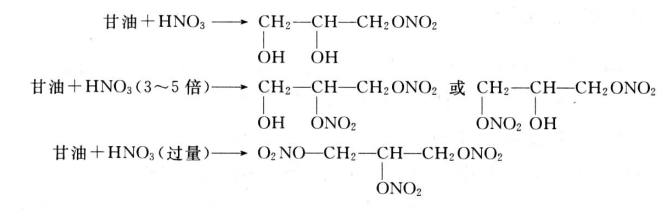
2.2.5 Nitrification reaction
Glycerol reacts with dilute nitric acid or glycerol reacts with nitric acid to form a mixture of mononitro compounds or mononitro and dinitro compounds. Two nitro glycerin was produced when glycerol was acted on 3-5 times of nitric acid. Trinitro glycerin is produced when excessive nitric acid reacts with glycerol or when a mixture of sulfuric acid and nitric acid reacts with glycerol.