Glycerol (also known as glycerin) is a major byproduct in the biodiesel manufacturing process. In general, for every 100 pounds of biodiesel produced, approximately 10 pounds of crude glycerol are created. As the biodiesel industry is rapidly expanding, a glut of crude glycerol is being created. Because this glycerol is expensive to purify for use in the food, pharmaceutical, or cosmetics industries, biodiesel producers must seek alternative methods for its disposal. Various methods for disposal and utilization of this crude glycerol have been attempted, including combustion, composting, anaerobic digestion, animal feeds, and thermochemical/biological conversions to value-added products. The objective of this article is to provide a general background in terms of waste glycerol utilization.
Characterizations of Glycerol Waste
Crude glycerol generated from biodiesel production is impure and of little economic value. In general, glycerol makes up 65% to 85% (w/w) of the crude stream . The wide range of purity values can be attributed to different glycerol purification methods or different feedstocks used by biodiesel producers. For example, Thompson & He (2006) have characterized the glycerol produced from various biodiesel feedstocks. The authors found that mustard seed generated a lower level (62%) of glycerol, while soy oil had 67.8 % glycerol, and waste vegetable oil had the highest level (76.6 %) of glycerol.
Methanol and free fatty acids (soaps) are the two major impurities contained in crude glycerol . The existence of methanol is due to the fact that biodiesel producers use excess methanol to drive the chemical transesterification to completion, and do not recover all the methanol. The soaps, which are soluble in the glycerol layer, originate from a reaction between the free fatty acids present in the initial feedstock and the catalyst (base).i.e.,
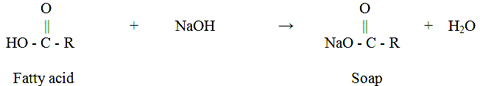
In addition to methanol and soaps, crude glycerol also contains a variety of elements such as calcium, magnesium, phosphorous, or sulfur. Thompson & He (2006) reported that the elements present in the glycerol of different feedstock sources (such as canola, rapeseed, and soybean) were similar. Calcium was in the range of 3-15 ppm, magnesium was 1-2 ppm, phosphorous was 8-13 ppm, and sulfur was 22-26 ppm. However, when crambe (a perennial oilseed plant) was used as feedstock, crude glycerol contained the same elements, but at vastly different concentrations. Schröder & Südekum (1999) also reported the elemental composition of crude glycerol from rapeseed oil feedstock. Phosphorous was found to be between 1.05 % and 2.36 % (w/w) of the crude glycerol. Potassium was between 2.20 % and 2.33%, while sodium was between 0.09% and 0.11%. Cadmium, mercury, and arsenic were all below detectable limits.
The crude glycerol derived from alkali-catalyzed transesterification usually has a dark brown color with a high pH (11-12). When pH is adjusted to a neutral range, soaps will be converted into free fatty acids, as shown in the following equation
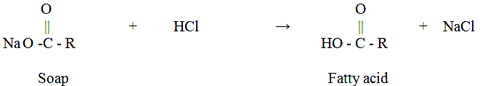
The free fatty acids in the crude glycerol stream results in a cloudy solution. After settling for a period of time, this cloudy solution will be separated into two clear phases, with the top layer being the free fatty acid phase, and bottom layer the glycerol phase.
New Uses For Glycerol Waste
There are various outlets for disposal and utilization of the crude glycerol generated in biodiesel plants. For large scale biodiesel producers, crude glycerol can be refined into a pure form and then be used in food, pharmaceutical, or cosmetics industries. For small scale producers, however, purification is too expensive to be performed in their manufacturing sites. Their crude glycerol is usually sold to large refineries for upgrading. In recent years, however, with the rapid expansion of biodiesel industry, the market is flooded with excessive crude glycerol. As a result, biodiesel producers only receive 2.5-5 cents/lb for this glycerol . Therefore, producers must seek new, value-added uses for this glycerol.
There have been many investigations into alternative uses of crude glycerol. Combustion, composting, animal-feeding, thermo-chemical conversions, and biological conversion methods for glycerol usage and disposal have all been proposed. Johnson and Taconi (2007) reported that combustion of crude glycerol is a method that has been used for disposal. However, this method is not economical for large producers of biodiesel. It has also been suggested that glycerol can be composted or used to increase the biogas production of anaerobic digesters . DeFrain et al. (2004) attempted to feed biodiesel-derived glycerol to dairy cows in order to prevent ketosis, but found that it was not useful.
Also, Lammers et al. (2008) studied supplementing the diet of growing pigs with crude glycerol. This study found that the metabolizable-to-digestible energy ratio of glycerol is similar to corn or soybean oil when fed to pigs. Therefore, the study concludes that “crude glycerol can be used as an excellent source of energy for growing pigs,” but also cautions that little is known about the impacts of impurities in the glycerol. Furthermore, Cerrate et al. (2006) have had some success with feeding glycerol to broiler chickens. Birds fed 2.5 % of 5% glycerin diets had higher breast yield than the control group, but the authors caution that there is still concern about methanol impurities in the glycerol.
Converting crude glycerol into valued-added products through thermo-chemical methods or biological methods is an alternative for utilizing this waste stream. It has been reported that glycerol can be thermochemically converted into propylene glycol , acetol , or a variety of other products. Cortright et al. (2002) have developed an aqueous phase reforming process that transforms glycerol into hydrogen. Virent Energy Systems is currently trying to commercialize this technology and claim that sodium hydroxide, methanol, and high pH levels within crude glycerol help the process .
For biological conversions of crude glycerol, the glycerol serves as a feedstock in various fermentation processes. For example, Lee et al. (2001) have used glycerol in the fermentation of Anaerobiospirillum succiniciproducens for the production of succinic acid. The fermentation of E. coli on glycerol leads to the production of a mixture of ethanol, succinate, acetate, lactate, and hydrogen . Glycerol can also be converted to citric acid by the yeast Yarrowia lipolytica. It has been reported that this organism produces the same amount of citric acid when grown on glucose or on raw glycerol . Rymowicz et al. (2006) found that acetate mutant strains of Y. lipolytica can produce high levels of citric acid while producing very little isocitrate. Furthermore, it has been shown that Clostridium butyricum can utilize biodiesel-derived glycerol to produce 1,3-propanediol (an important chemical building block with many industrial uses) in both batch and continuous cultures. During the fermentation process, the organism also produces byproducts of acetic and butyric acid. The researchers at Virginia Tech also developing algal fermentation processes to convert crude glycerol into high value omega-3 polyunsaturated fatty acids

