The chemical substance glycerol, Ch2oh, Choh, Ch2oh or glycerine as it is commonly known in commerce, is a colourless, odourless, viscid liquid having a sweet taste and a neutral reaction.
It is soluble in water in all proportions with evolution of heat, it is strongly hygroscopic and on exposure to the air as much as 50 per cent of its own weight of water is absorbed. It has powerful solvent properties, combining in this respect those of water and alcohol.
15°
Its sp. gr. at 15o/15o C.=1.26468.
It boils under 760 mm. pressure at 290° C. with slight decomposition, but under reduced pressure it distils unchanged, its b.p. at 50 mm. pressure being 210° C. and at 12.5 mm. 179.5° C. It is not volatile at ordinary temperatures, but on concentration of a solution of it in water at ordinary pressures glycerol volatilises with the water vapours at a temperature of about 160° C, corresponding to a concentration of about 70 per cent.
When strongly heated it rapidly loses water with the formation of acrolein, leaving a residue of poly glycerols, chiefly diglycerol C6H1405.
Commercially pure glycerine has a sp. gr. of 1.260 and, apart from about 2 per cent of water, it contains only the minutest traces of impurities.
This practically pure substance is produced by the distillation of crude glycerine, a by-product of the soapmaking and candlemaking industries.
Fats and oils, on saponification with caustic soda, yield a dilute impure solution containing about 5 per cent of glycerine, and this, on treatment and purification, yields a product containing 80 per cent of glycerine and 10 per cent of salts, known as soap lyes crude glycerine.
In the candle industry fats are hydrolysed by the autoclave, Twitchell, or similar process, yielding fatty acids and a solution of glycerine, and this solution on treatment and concentration yields saponification glycerine containing about 90 per cent of glycerine and a small percentage of salts and other impurities.
Approximate analyses of these two crude products are given below, so that the problem of distilling them for the production of glycerine in its purer forms may be understood.
| Crude glycerine. | Saponification glycerine. | |
| Glycerine T.A.V……………………………………….. | 81.28 | 86.47 |
| Specific gravity at 20°/20° C….. | 1.3010 | 1.2393 |
| Total residue at 160° C. | 12.45 | 0.80 |
| Ash …………………………………………………………………….. | 9.73 | 0.42 |
| Organic residue by difference ……………………………………… | 2.72 | 0.38 |
| Arsenic parts per million ……………………………………… | 2 | 1 |
The organic residue consists of polyglycerols, fatty acids, resinous and colouring matters not removed in the treatment of the lyes.
The ash in crude glycerine consists mainly of sodium chloride with traces of sodium carbonate and oxides of iron, aluminium, and silicon derived from the materials used in the preliminary treatment of the lyes.
The ash in saponification glycerine is usually calcium or barium sulphate, also derived from the treatment of the ” sweet water,” as the solution of glycerine obtained on splitting fats is usually termed.
Crude glycerine is a clear, bright, rather viscous liquid, with a colour varying from orange-red to dark brown, while saponification glycerine is less viscous and lighter in colour.
The apparatus used for distilling each of these two products is identical, but owing to the larger amount of solids in crude glycerine greater precautions have to be taken to prevent overheating and the distillation is somewhat slower.
As by far the greatest bulk of glycerine produced comes from the soap lyes crude glycerine, the plant described will be that particularly applicable to the distillation of crude glycerine, though it is equally suitable for saponification glycerine.
Glycerine can only be distilled without loss under reduced pressure, and all the plants here described are worked under vacuum and free steam is invariably used to assist the distillation.
The earliest patents taken out for the distillation of crude glycerine in this country date from about 1881, as it was not until 1879 that the successful production of crude glycerine from soap lyes was effected on the manufacturing scale, and from this date onwards the efforts of the designers of glycerine distilling plants have been directed to the three points (1) Prevention of overheating the substance, and consequent loss of glycerine;
(2) Condensation of the greatest amount of strong glycerine ;
(3) Economy in the use of fuel; and the various plants described below indicate the successive improvements that have been made in these directions, and latterly chiefly in the economy of fuel. The prevention of overheating and consequent loss due to the formation of polyglycerols and other products of the destructive distillation of crude glycerine were at once overcome when stills heated by means of steam at a definite and constant temperature took the place of fire-heated stills.
Fire-heated stills are now quite obsolete, but it may be of interest to describe one exampleso that the progress that has been made in glycerine distillation plant may be emphasised subsequently.
The illustration below (Fig. 181) shows the general arrangement of the plant, a is a cylindrical still with a flat bottom mounted on the brickwork setting b, which is fitted with a grate for the coal fire, the flames from which play on to the bottom of the still and around the sides below the level of the glycerine in the still, a current of free steam superheated in the same fire and controlled by the valve c bubbles through the liquid in the still in the form of fine jets.
The glycerine and water vapours pass through the still-head d and any entrainment is caught in the catchall e, and returns to the still by means of the pipe f, the vapours passing on to the series of air-cooled condensers Gl, g2 to G6 ; the vapours enter each condenser at the bottom and, emerging from the top, are carried to the bottom of the next condenser by means of the pipes h1, h2 to h5.
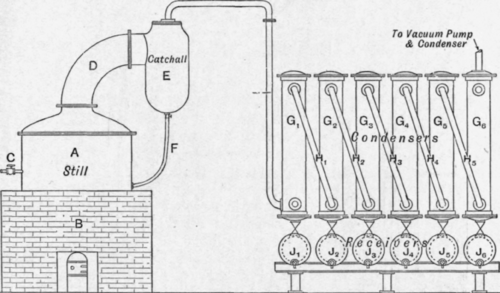
Fig. 181. – Glycerine distillation plant. Fire-heated still.
Under each condenser is a receiver Jl, J2 to J6, and in these the condensed glycerine is collected; from the first two the best quality is obtained, the quality gradually deteriorating and the glycerine becoming weaker towards the end condenser where the last of the glycerine should be condensed with much of the volatile impurities.
A water condenser and vacuum pump is connected to the top of the last condenser.
The loss of glycerine in such a plant is very heavy when crude glycerine is distilled ; the salt soon separating out and settling on the bottom of the still causes local overheating and decomposition of the glycerine with the formation of acrolein and other products
With saponification glycerine a fairly good yield can be obtained and the distilled glycerine is of good quality, though very great care is necessary in controlling the fire and the free steam, any excess of the latter causing the glycerine to froth over the still-head and contaminate the distillate already collected.
A much more efficient type of distillation plant that is largely used in this country is that of Van Ruymbeke, the distinctive characteristic of which is the heating of the glycerine in the still by means of high pressure steam circulating through a coil and the superheating of the expanded free steam by means of the steam from the close coil after it has passed through the still.
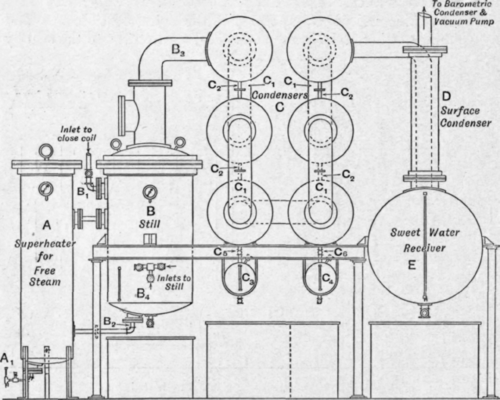
Fig. 182. – Glycerine distillation plant. Van Ruymbeke system.
The plant (Fig. 182) consists of a steam superheater A, a still b, a set of condensers c, with suitable receiving vessels for collecting the condensed strong glycerine, a water-cooled surface condenser d connected to a large receiving vessel e in which the weak glycerine or “sweet wate ” collects, and which in turn is connected with a condenser and vacuum pump capable of maintaining a high vacuum.
The still b is heated by means of steam passing through a spiral coil of many turns which extends from the inlet B1 to the outlet b2, and then through a well insulated pipe to the superheater A, and the current of steam is so regulated that the pressure of the steam, and therefore also the temperature, is kept the same in the superheater as that of the steam entering the close coil; this pressure varies between 140 and 2001b. per square inch, and the condensing surface of the plant is designed for a particular pressure of steam which should not be varied greatly. Under vacuum glycerine is not decomposed at a temperature of 200° C, and the greatest economy in working is obtained by distilling at the highest possible temperature. In practice, steam of 180 lb. pressure is found very suitable as it will give a still temperature of 195° C, and references in the description of this particular type of glycerine distilling plant will be made to one designed for use with steam of a pressure of 180 lb. per square inch. The free steam superheater is fitted with a coil spirally wound inside the vessel, and steam from the same main as that providing the close steam is admitted into this coil through a valve A1 Half a turn of a 1/2 in. valve is sufficient to admit the requisite quantity of steam into the 2 in. coil in the superheater. The loss of heat on expansion is made up by the high pressure steam in the superheater surrounding the coil, and, finally, when the steam reaches the end of the coil at the top of the vessel, although it is at a low pressure, it has the same temperature as the high pressure steam heating the contents of the still.
The function of the close coil in the still is to heat the contents of the still to the distilling temperature, and to maintain it at this temperature when distillation starts by supplying the heat required to make up for radiation losses from the still and that absorbed in vaporising the glycerine, and the function of the open steam is to keep the mass agitated, to supply the difference in pressure between the pressure at which the still is working and the vapour pressure of the glycerine at the temperature of the still, and to increase the velocity of the vapours so that they will be carried through the still-head and into the condensers before they can condense and drop back into the still.
In all cases of distillation carried out with the aid of currents of steam it is advisable that the temperature of the steam should not be less than that of the liquid undergoing distillation, otherwise priming occurs due to the rapid expansion of the lower temperature steam entering the hot liquid in the still; and on the other hand, if the steam is hotter than the liquid there is a loss of efficiency and also danger of decomposing the liquid. In the ingenious manner just described both these dangers are overcome, and the distillation is controlled by the extremely simple method of noting that the steam pressure in the superheater is the same as that of the steam entering the close coil.
The condensers c which are connected to the still by the pipe b3 are cylindrical drums connected to one another by the vapour pipes C1 and by the smaller diameter pipes c2, through which the condensed glycerine flows downwards, collecting in the receiving vessels c3 and c4.
These condensing drums can be arranged, as shown in the section of the plant illustrated, in two tiers, or they can be arranged in a horizontal row, in which case it is necessary either to have a receiving vessel connected beneath each drum, or, as is more common, to have no receiving vessels at all, but to allow the condensed glycerine to collect in the condensing drums until the distillation is complete, when all the drums can be emptied into tanks placed on the ground beneath them.
There are advantages in both methods, and it is largely a question of available space as to which arrangement is adopted. The vertical arrangement has the advantage of utilising the full condensing surface of the drums for the whole period of the distillation; it is the most compact, and is to be recommended where floor space is limited and head room is available.
The horizontal arrangement has the disadvantage of a gradually reduced condensing surface due to the drums partially becoming filled with condensed glycerine, but there is the advantage of fractionating the glycerine into six or more fractions, depending on the number of condensing drums employed, instead of obtaining two fractions only in the case of the vertical arrangement.
On the whole where the head room is available the advantage lies with the vertical arrangement, as it is seldom necessary to fractionate the strong glycerine; in practice all the fractions are mixed together for concentration to produce dynamite glycerine, or for a second distillation to produce chemically pure glycerine. After passing through the series of air-cooled condensers where 85 per cent to 90 per cent of the distillate should be condensed, the vapours pass through the tubular condenser d, which is cooled by a stream of water passing through it on the outside of the tubes, the glycerine vapours passing through the tubes where they condense, together with most of the steam employed in assisting the distillation, and collect in the sweet water receiver e. These sweet waters are therefore a weak solution of glycerine containing all the volatile condensable impurities of the crude glycerine.
The uncondensed vapours then pass to a barometrical condenser where the last traces of water vapour are condensed and the incondensable vapours, air, etc., are removed by means of a vacuum pump. Other types of condensers and vacuum pumps may, of course, be employed.
Having described the plant, we can now pass to an account of the operation of distillation. The first operation is to get the whole plant under vacuum, and the pump is started and the air evacuated. A good dry air pump will maintain an absolute pressure, while the still is working, of one and a half inches to two inches of mercury, and for efficient work it is necessary to have this vacuum.
Crude glycerine is then drawn into the still by means of a pipe connected to the valve b4, and steam let into the close coil by opening the valve b1 When the glycerine has attained the temperature of the steam, free steam is admitted by opening the valve A1.
The amount of crude glycerine fed into the still is sufficient to cover the perforated pipe from which the free steam issues, and as the distillation proceeds glycerine is fed in more or less continuously at a rate equal to the rate of distillation until the solids and non-volatile constituents of the crude glycerine have accumulated to a volume approximately equal. to the original charge fed into the still. In practice it amounts to the total charge being about seven times the weight of the original amount fed into the still.
On admitting the free steam, distillation starts at once and proceeds rapidly and uniformly; the amount of free steam used varies considerably, but in an efficient plant it should be about equal to the weight of crude glycerine in the charge.
The distilled glycerine collects in the receivers c3 and c4, about twice as much in the former as in the latter, and as they become full they are emptied by closing the valves c5 and c6, which connect them to the condensers, admitting air to the receivers through a small cock, and opening the valves at the bottom. When emptied, the valves at the bottom and the small air cocks are closed, and the valves c5 and c6 are carefully and very gradually opened until the pressure in the receivers and the rest of the plant is equalised.
The specific gravity of the glycerine collected in the first receiver c3 is 1.260 or higher, and that in the second receiver c4 about 1.240, giving an average specific gravity for the whole distillate of about 1.253 = 95 per cent glycerol. This is afterwards concentrated in a vacuum pan to 1.260 specific gravity or 98 per cent glycerol, which is the standard concentration of commercial glycerine.
The sweet water collected in the receiver e is about equal in weight to the weight of the whole charge of crude glycerine, and contains approximately 10 per cent of glycerine and most of the volatile impurities in the crude. This is concentrated in a separate vacuum pan, and then undergoes further distillation and fractionation.
When no more glycerine will come over the distillation is stopped, all steam turned off, the pump stopped and air is slowly admitted to the plant until it is under atmospheric pressure. The residue in the still, or “glycerine foots,” is run out as a viscous tarry mass ; the still is washed out with boiling water, and this water is added to the foots, which undergo subsequent treatment, concentration, and further distillation for the recovery of the glycerine they contain.

